
The above was created on paper using the microemuslions reported in Chem. Commun. as ink and illuminated using a Ultraviolet lamp (wavelength = 365 nm)
Functional organic liquids (FOLs) are unique, in that they can flow and act as a solvent, while still being able to act as semiconductors or retain efficient emission properties.
FOLs can be formed when long branched alkyl chains, are added to key functional organic moities such as pyrene, fullerene-C60, anthracene (etc.). The bulky and flexible groups pack poorly and disrupt π–π interactions between the functional part of the molecule, leading to a significant reduction in the melting point and consequently FOLs are oils at room temperature.
In a recently published (open access!) paper (Chem. Commun., 2016,52, 7344-7347) we’ve looked into the possibility of forming oil-in-water microemulsions with a newly developed pyrene-based FOL , which is shown below.
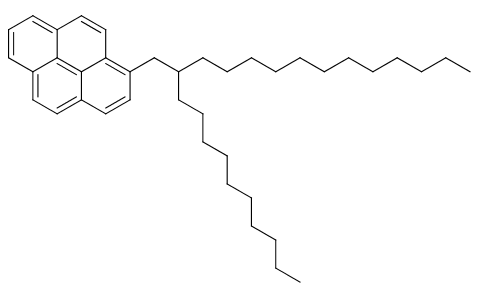
Liquid alkyl-pyrene derivative developed in the study
Why oil-in-water microemuslions? Well, asides from the basic fact that this had not been done before (and could therefore lead to unforseen benefits), the liquid pyrene shown above has a very high fluorescent quantum yield (i.e. in comparison to the amount of light absorbed, the amount of light emitted is pretty high – around 65%). Comparmentalising and dispersing this oil in water may lead to an effective contrast-enhancing agent for fluroescence microscopy, which is commonly used by biologist and medics to look at cellular structure (etc.). For some idea of the kinds of images that can be taken using fluorescence microscopy, see the wikipedia page.
To summarise the results, we successfully formed microemulsions using the liquid pyrene, a surfactant (C12E6) and used small-angle neutron scattering to investigate their structure (droplets; short cylinders). Inside the droplets, the bright fluorescence of the pyrene derivative was retained. Interestingly, the emission colour changed with droplet size, which can be easy altered by the concentration of the 3 components.
While we’ve yet to show that this new system can be used in imaging, other researchers have used nanoemulsions containing fluorescent dyes or quantum dots for contrast enhancement. The FOL-in-water microemulsions may have benefits over those system in terms of biocompatibility, long-term stability, ease of formation or brightness. We will continue to investigate these possibilities in further work!
Link to paper (Open Access, published under a CC-BY license):
http://pubs.rsc.org/en/content/articlelanding/2016/cc/c6cc01517d#!divAbstract


![20140605_110713[1]](https://martinhollamby.files.wordpress.com/2014/06/20140605_1107131.jpg?w=225&h=300)





